Difficulties in getting pregnant
Trial location
- Gender Female
- Age from 18 up to and including 40
- BMI from 15 up to and including 35
- Calculate your BMI here
Difficulties in getting pregnant
The ULTRA study is being carried out by several European centres in order to evaluate an innovative medical device developed by May Health for the treatment of infertility in women suffering from Polycystic Ovary Syndrome (PCOS), also called Stein-Leventhal syndrome.
PCOS is a hormonal imbalance which leads to the appearance of various symptoms (acne, excessive hair growth, excess weight) which may vary from one person to another. Patients generally have larger ovaries than other women and a high number of ovarian follicles, generally accompanied by the lack or absence of ovulation which causes infertility (inability to get pregnant). More than 1 woman in 10 suffers from this syndrome.
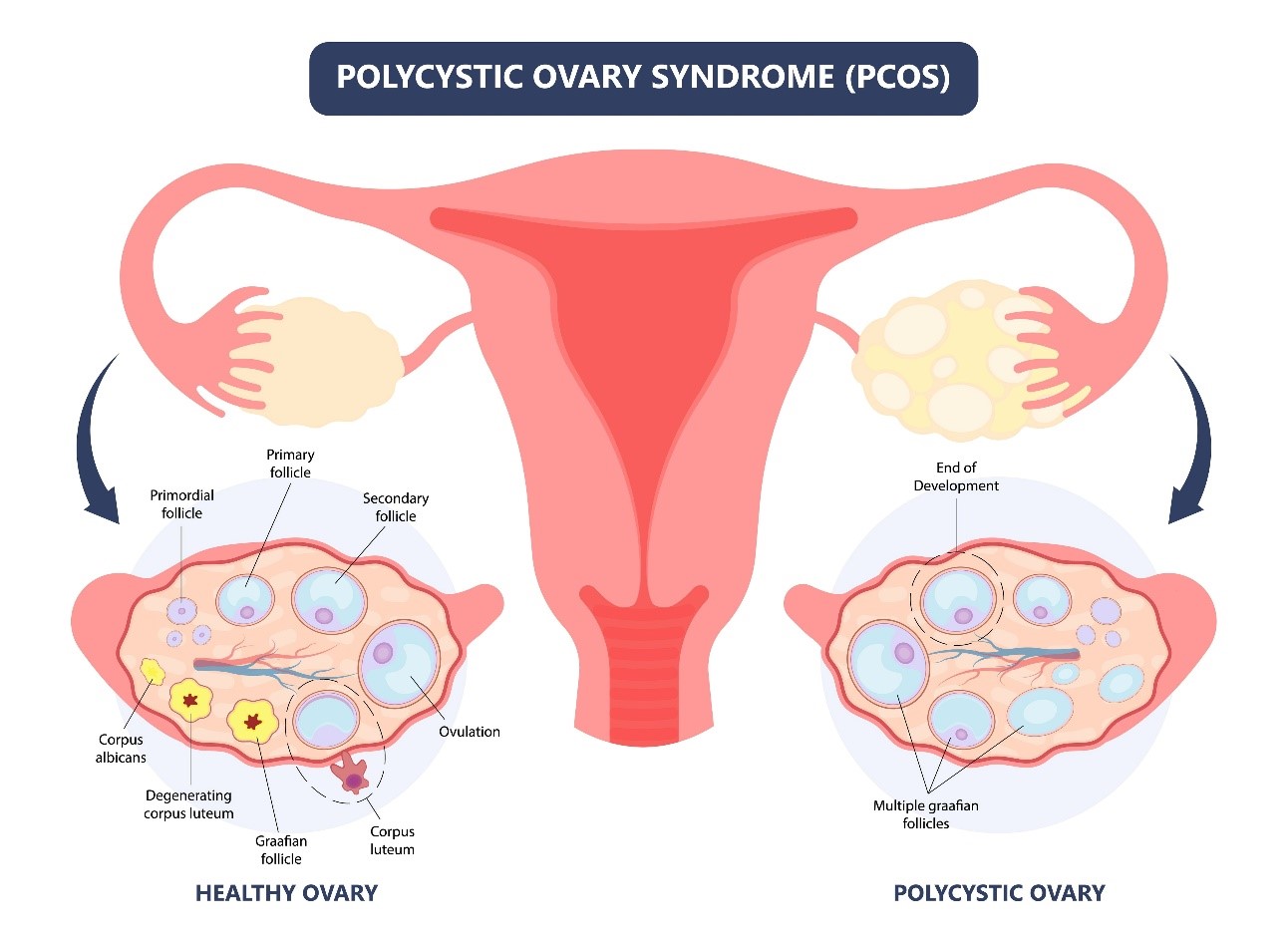
Several treatments exist to stimulate ovulation using drugs. They are not always effective and may have side effects. In addition, they do not treat the other symptoms linked with PCOS. The May Health procedure has been developed to help PCOS patients to experience a more natural and less medicalised ability to become pregnant
It has been shown that the reduction in volume of the ovaries may contribute to restoring the hormone balance which enables natural ovulation to be re-established. The May Health solution has been developed to reduce the volume of the ovaries by means of a short procedure taking less than 1 hour, without a general anaesthetic. The procedure is carried out only once and uses a non-surgical minimally invasive technique, via the vagina, similar to IVF (In-Vitro Fertilisation).
The patient is positionned on the table, lightly sedated. The medical device, looking like a long needle is installed on a transvaginal ultrasound probe. The ultrasound probe is inserted into the patient’s vagina to allow ultrasound visualisation of the ovaries. Once in proximity with one of the ovaries, the needle is inserted, and a small catheter is deployed. The tissue to be destroyed is heated by the catheter for a a minute at a time, in several places within the ovary. The procedure may be repeated on the second ovary. The catheter and needle are then withdrawn and the patient would be able to go home after a few hours.
Who may take part in this clinical study?
You are eligible for the ULTRA study if:
- You are aged between 18 and 40 years
- You have been diagnosed with Polycystic Ovary Syndrome (PCOS) by a health professional
- You plan to get pregnant and are encountering difficulties in getting pregnant
- You have tried an initial ovarian stimulation treatment (Clomiphene citrate or Letrozole) without success: you did not ovulate at least during your last 2 cycles. You are in a couple relationship with a man who has no fertility problems
What will happen if you decide to participate in this clinical study?
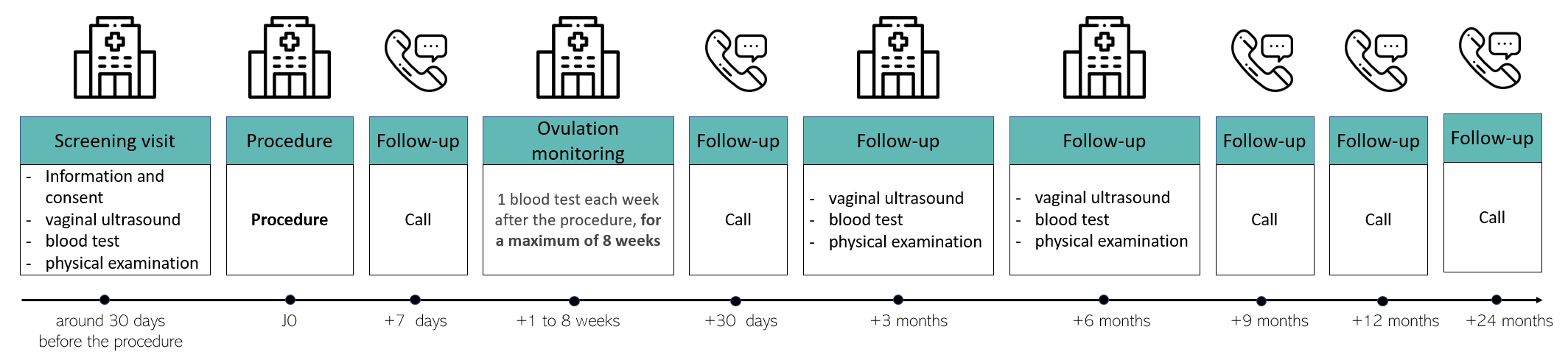
If you take part in this clinical study, you will have to come to the hospital for several monitoring visit over a total period of 24 months (see image schedule below).
- The first visit will confirm your inclusion in the clinical study. Examinations will be carried out: blood test, vaginal ultrasound scan and physical examination
- The procedure will be carried out at the second visit
- You will then be asked to come back each week for up to 12 consecutive weeks to carry out a blood test to verify whether your natural ovulations are reappearing.
- At three and six months after the procedure, you will have to come back to the hospital to undergo the same examinations as at your first visit in order to compare the results
- During your participation, telephone calls with the medical team will be scheduled.
You will benefit from the following:
-
The procedure and monitoring examinations of the clinical study, free of charge
-
You will be closely monitored throughout the clinical study by a centre with expertise in infertility linked to PCOS
-
Your travelling expenses will be reimbursed.
-
You will participate in PCOS awareness/ advancing science for you and or other women affected by PCOS
How to participate?
If you wish to participate in this clinical study, please register on this internet site by filling in the questionnaire below. If you meet the main selection criteria, you will be contacted to discuss the clinical study and your interest in participating in it in more detail.
Completing this questionnaire does not commit you to participate in the study. You will still have time to discuss your participation with your loved ones, your attending physician, and the study medical team as you wish.
A clinical study means a scientific study that consists in observing the effect, in humans, of a new drug/product/examination/treatment or an existing drug/product/examination/treatment. By means of clinical studies, doctors are able to find new and better methods for preventing, detecting, diagnosing, controlling and treating diseases. All clinical studies are closely monitored and regulated to guarantee the well-being of the participants. Participating in a clinical study is entirely voluntary and will in no way affect the quality of care that you would normally be given.
What is experimental about this study?
The device and the treatment provided by the May Health system are experimental in Europe, in that they have not yet been approved by the regulatory authorities.
Has this medical device already been used in individuals?
The ULTRA system has already been used in this clinical study, which has been underway since 2019.
How many people will participate in this study? For how long will they take part?
This study will recruit up to 30 participants with a monitoring period of 24 months. Several hospitals are participating in the study, in Belgium, France and the United Kingdom.
What do I need to know about this study? The examinations and procedures carried out during the study will not cost you anything You will be closely monitored throughout the study by a centre with expertise in infertility linked to PCOS It is essential that you attend all the appointments scheduled for the study. You may withdraw from the study at any time and for any reason. This will not in any way affect the quality of care that you might be given. You can talk to the study team to ask them questions or share your concerns with them at any time.
May Health (previously known as AblaCare) is the company taking the initiative for this research and assuming the responsibilities and financing. Its registered office is based in Paris, France. To find out more: 9 rue d’Enghien, 75010, Paris, France
May Health has developed an innovative procedure to treat infertility in PCOS patients (Polycystic Ovary Syndrome), to enable them to experience a natural and less medicalised access to pregnancy.

 Argentina
Argentina Australia
Australia Balgarija
Balgarija België
België Canada
Canada Česko
Česko Chile
Chile China (中国)
China (中国) Colombia
Colombia Danmark
Danmark Deutschland
Deutschland England
England España
España France
France Ireland
Ireland Italiana
Italiana Lietuva
Lietuva Magyarország
Magyarország Nederland
Nederland New Zealand
New Zealand Österreich
Österreich Polska
Polska Schweiz
Schweiz Singapore
Singapore Slovenija
Slovenija Slovensko
Slovensko Suomi
Suomi Sverige
Sverige United States
United States Israel
Israel